Abstract
Background Therapeutic advances have increased life expectancy for patients with RRMM. As survival has improved, SPMs have become an increasingly important safety consideration for RRMM patients. Prior studies have demonstrated that treatment with alkylators, autologous stem cell transplantation (ASCT), and immunomodulatory drugs (IMiDs), specifically lenalidomide, are associated with increased SPM risk. Patients who are TCE (exposure to ≥1 IMiD, ≥1 proteasome inhibitor [PI], and ≥1 anti-CD38 monoclonal antibody [mAb]) represent an increasingly large subgroup of RRMM patients. The objective of this study was to describe the incidence of SPMs in patients with TCE RRMM. These data may inform development and selection of novel treatments for these patients.
Methods Data were from two large US health-insurance claims databases: the Optum Clinformatics® Data Mart and the IQVIA PharMetrics® Plus Health Insurance Claims database. TCE RRMM patients were selected if they had ≥1 confirmed diagnosis of multiple myeloma (MM) on or after December 1, 2015 (date of approval by the US Food and Drug Administration of 1st anti-CD38 mAb for RRMM) and evidence of being TCE. The first line of therapy (LOT) after becoming TCE was identified ("index LOT") and the date of initiation of this LOT was defined as the "index date". Patients who experienced SPMs prior to the index date were excluded. SPMs were identified based on having at least one inpatient or two outpatient diagnoses (of the same cancer type) of invasive malignancies or myelodysplastic syndromes (MDS) other than MM, Waldenstrom macroglobulinemia, plasma cell leukemia, non-melanoma skin cancers, or primary bone malignancies that were preceded by a diagnosis of bone metastases. The incidence of SPM was calculated per 100 person years (PY) of follow-up, considering only the first SPM for each patient. Cumulative incidence of SPM with death as competing risk was calculated for patients in Optum database (data on mortality was unavailable in the PharMetrics database).
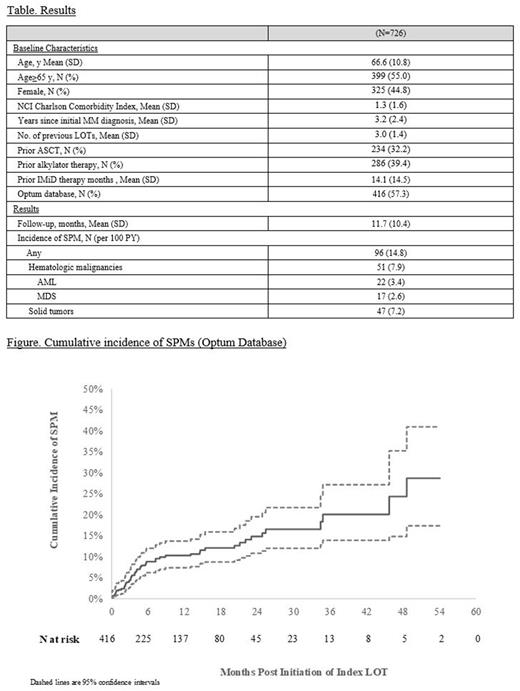
Results A total of 1,110 TCE RRMM patients were identified in the two databases, of whom 726 had no evidence of SPM prior the index date (Table). These 726 patients had a mean age of 66.6 y; 45% were female, with a mean of 3 prior LOTs and 3.2 years since diagnosis. Over a mean (SD) follow-up of 11.7 (10.4) months, 96 patients (14.8 per 100 PY) experienced a new SPM, including 47 (7.2 per 100 PY) with solid tumor and 51 (7.9 per 100 PY) with a hematologic malignancy. The latter included 22 (3.4 per 100 PY) with acute myeloid leukemia (AML) and 17 (2.6 per 100 PY) with myelodysplastic syndrome. Among patients in the Optum database (N=416), the cumulative incidence of SPM (95%CI) was estimated to be 10.3% (7.4% , 13.7%), 14.9% (10.8% , 19.6%), and 20.1% (13.9% , 27.2%) at 12, 24, and 36 months, respectively (Figure).
Conclusions The incidence of SPM in TCE RRMM patients is clinically significant. Many SPMs in these patients are potentially serious malignancies, including AML and MDS. There is a need for effective treatments for TCE RRMM that are not associated with increased risks of SPMs.
Disclosures
Ge:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Archambault:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Delea:GRAIL: Research Funding; InterMune: Research Funding; Ionis: Research Funding; Karius: Research Funding; Leo Pharmaceuticals: Research Funding; MinervaX: Research Funding; Moderna: Research Funding; Myovant Sciences: Research Funding; Oncimmune: Research Funding; Otsuka: Research Funding; Policy Analysis Inc.: Current Employment, Other: equity ownership; Novartis: Research Funding; Sanofi: Research Funding; Regeneron: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Research Funding; Akcea: Research Funding; Akebia: Research Funding; Amgen: Research Funding; Cerevel: Research Funding; Dynavax: Research Funding; Eidos: Research Funding; Global Blood Therapeutics: Research Funding; GlaxoSmithKline: Research Funding; Alexion: Research Funding; AbbVie: Research Funding; Fishawack Health: Other: equity ownership; Vertex: Research Funding; Tactile Health: Research Funding. Rodriguez Lorenc:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Harnett:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; Pfizer Inc.: Current equity holder in publicly-traded company. Moynahan:Policy Analysis Inc., a wholly owned subsidiary of Fishawack Health: Current Employment. Weycker:Tactile Health: Research Funding; Fishawack Health: Other: equity ownership; Policy Analysis Inc.: Current Employment, Other: equity ownership; Global Blood Therapeutics: Research Funding; Dynavax: Research Funding; GlaxoSmithKline: Research Funding; Eidos: Research Funding; InterMune: Research Funding; GRAIL: Research Funding; Alexion: Research Funding; Amgen: Research Funding; Abbvie: Research Funding; Akebia: Research Funding; Akcea: Research Funding; ADC Therapeutics: Research Funding; Cerevel: Research Funding; Vertex: Research Funding; Takeda: Research Funding; Seattle Genetics: Research Funding; Sanofi: Research Funding; Regeneron: Research Funding; Pfizer: Research Funding; Otsuka: Research Funding; Oncimmune: Research Funding; Novartis: Research Funding; Myovant Sciences: Research Funding; Moderna: Research Funding; MinervaX: Research Funding; Leo Pharmaceuticals: Research Funding; Karius: Research Funding; Ionis: Research Funding. Houvras:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kroog:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wu:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Hampp:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Ma:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal